About Us
Dr. Geetanjali’s Healthcare Center pvt.ltd is a NABL-accredited Central Pathology Laboratory, providing specialized pathology diagnostic services tailored for clinical trials. We are committed to delivering the highest standards of quality, accuracy, and compliance in every sample we process. With a strong focus on supporting clinical research and drug development, we cater to the unique needs of pharmaceutical companies, biotechnology firms, and Contract Research Organizations (CROs) across India. Our comprehensive central lab services are designed to streamline study workflows, enhance data integrity, and accelerate timelines — from Phase I to Phase IV clinical trials.
🧪 Quality
100% commitment to delivering accurate, validated results
💡 Innovation
Constantly evolving with the latest diagnostic technologies
🎯 Goal-Oriented
Protocol-aligned services focused on trial outcomes
🛡️ Integrity
Operating with transparency and ethical practices
🤝 Customer-Centric
Prioritizing client satisfaction and responsiveness
👥 Teamwork
Collaborative execution for seamless delivery
Why Choose Us as a Central Pathology Laboratory for Clinical Trials?
- NABL Accredited & Fully GCP/GLP-Compliant
- Proven experience in Phase I–IV & Post-Marketing Trials
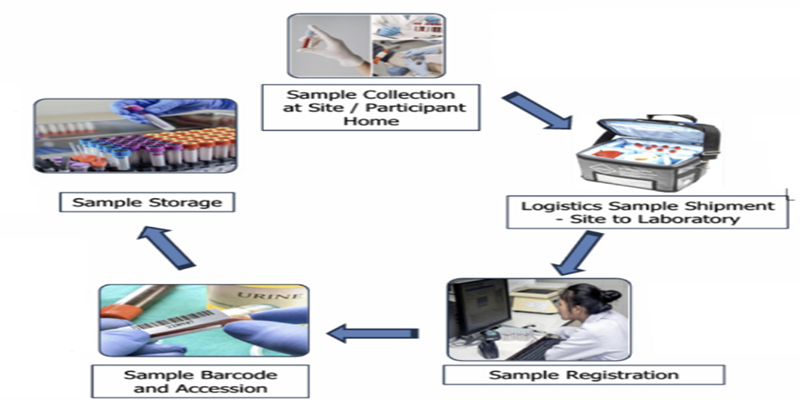
- Robust Global Sample Logistics with temperature control
- Rapid Turnaround and Real-Time Data Access
- Therapeutic expertise in Oncology, Hormones , Vaccines , Cardiology , Neurology, Immunology , Gastroenterology, Urology, Infectious Diseases , Metagenomics , Nutritional and Herbal studies
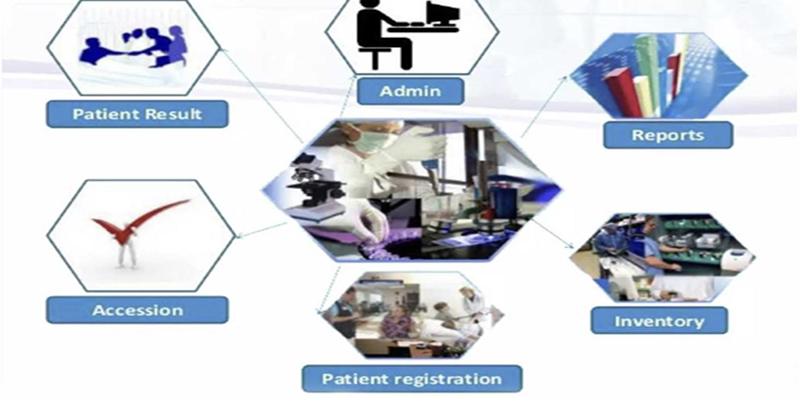
Services for Clinical Trials
- Dedicated Trial Coordinator from Central Lab for efficient coordination
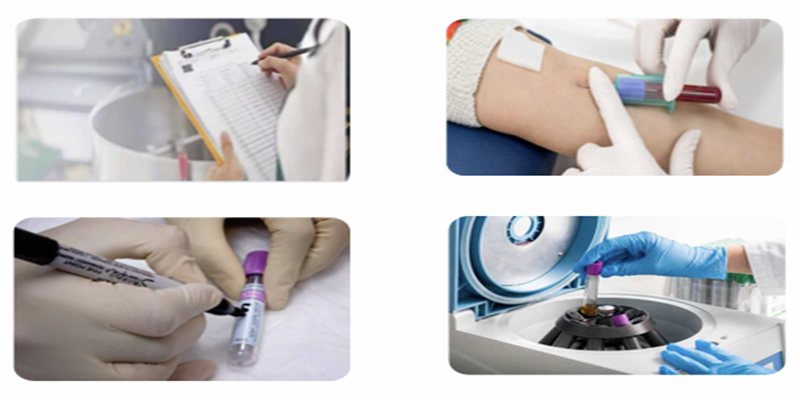
- Subject & Visit Specific Sample Collection Kits
- Temperature-Controlled Transport with chain-of-custody tracking
- IATA-Compliant Shipping ensuring international regulatory adherence
- Automated Sample Accessioning & LIMS Integration
- Real-Time Web Portal Access for sample tracking & reports
- Long Term Sample Storage Facility
- 0+ Clinical Trials Supported
- 0+ Tests Processed Annually
- Expertise in Global Sample Handling and documentation
- Coverage across multiple therapeutic areas
- Vast experience with Regulatory, Academic & Commercial Trials
- NABL Accreditation and ISO Certificationswith robust internal quality management system for quality excellence
- Compliant with GCP (Good Clinical Practice) and GLP (Good Laboratory Practice)
- IATA-Certified Logistics for international and domestic sample shipments from site to central lab
- Regular Internal Quality Audits to ensure compliance and continuous improvement
- Inspection-Ready for sponsor audits and regulatory visits


















.jpeg.jpg)